Abstract
Background
Post solid organ transplant Burkitt lymphoma (PSOT-BL) is a rare form of monomorphic post-transplant lymphoproliferative disease (M-PTLD). Outcome data in pediatric patients are limited and guidelines on optimal treatment in PSOT-BL are lacking.
Methods
Retrospective study and analysis of clinical characteristics, treatment, and outcome data from patients diagnosed with PSOT-BL at age ≤ 21 years (y) in 11 US pediatric transplant centers.
Results
We identified 28 patients transplanted between 1993-2016 who developed PSOT-BL. Median age at organ transplantation and at diagnosis of PSOT-BL was 2.9y (range, 0.1-14) and 7.5y (range, 7-17), respectively, with median interval from transplant to PSOT-BL diagnosis of 3.8y (range 2-7). Heart and liver were the most frequently transplanted organs (n=14, 11), while bowel, kidney, and lung were each transplanted in one patient. Immunosuppressive therapy at the time of PSOT-BL diagnosis was tacrolimus alone (n=9), or in combination with mycophenolate (n=7), azathioprine (n=6), cyclosporin (n=3), or prednisone (n=3). None were receiving an mTOR inhibitor at time of PSOT-BL diagnosis.
Six, twelve, and ten patients corresponded to Murphy stages II, III, and IV, respectively. Lactate dehydrogenase was elevated in 23 patients (82%) and was > 2x upper limit of normal in 14 (50%). All tumors were pathologically consistent with BL; MYC FISH testing was positive in 10/10 tumors tested, while 21/23 tumors (90%) had detectable Epstein Barr Virus.
Rituximab-containing regimens were given to 25/28 patients (93%). These treatment regimens were as follows: rituximab alone (n=2), low dose cyclophosphamide, prednisone, and rituximab (CPR) (n=10), Burkitt lymphoma specific therapy: (FAB/LMB chemotherapy (n=3) or FAB/LMB therapy with rituximab (n=8)), reduced dose FAB/LMB (n=2), diffuse large B-cell lymphoma (DLBCL) regimens including EPOCH-R (n=2) and R-CHOP (n=1).
Treatment modifications/reductions were infrequent. Methotrexate dose modification and/or omission were required in four patients due to renal dysfunction (n=3) and ascites (n=1). Doxorubicin was electively eliminated in one cardiac transplant recipient due to concern for cardiotoxicity and dose-reduced in another due to prolonged myelosuppression. There was no treatment related mortality.
There were two episodes of rejection, one was a liver rejection that was successfully treated without graft loss, the other occurred after achieving partial response (PR) to 3 cycles of CPR, required intensive immune suppression, and was followed by PTLD/DLBCL and death from PTLD progression. There were two additional cases of a second PTLD/DLBCL. One died of PTLD progression nine months after developing a second PTLD and the other is alive in complete remission (CR) 17 months after diagnosis of a second PTLD.
Of the two patients treated with rituximab alone, one had no response and the other relapsed. Both were subsequently treated with BL chemotherapy and are alive in CR 15- and 7-years following BL diagnosis, respectively.
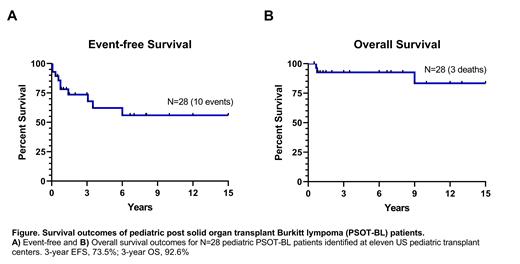
For all study patients over a median of 6.8y follow-up (range, 0.5-17), 3-year event-free survival (EFS) was 73.5% and 3-year overall survival (OS) was 92.6%, see figure. Three patients died of Burkitt lymphoma (all had been treated with CPR, experienced treatment failure and required treatment escalation to intensive BL chemoimmunotherapy but died of progressive disease). Of the 11 patients who received Burkitt lymphoma intensive therapy, all are alive in CR. One had progressed on treatment but achieved CR with further treatment intensification. Another developed a second PTLD/DLBCL, with subsequent CR.
Conclusion
This collaborative study represents the largest pediatric series of PSOT-BL reported to date. In this study patients with PSOT-BL treated with intensive BL directed therapy appear to have comparable outcome to immunocompetent pediatric patients with BL.
Orjuela: ATARA Pharmaceuticals: Other: Scientific Advisory Board.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal